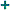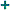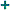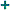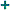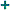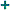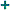e /e/
|
atoms; chemical elements [atomic physics] ↞ dte electrons dv hadrons ≈ DDC 541 |
e95 /eɲɔlpɔ/ []
|
with A; ampere electric current; I ↞ daml electric energy |
e95b /eɲɔlpɔba/
|
negative charge |
e95c /eɲɔlpɔʃa/
|
no charge |
e95m /eɲɔlpɔma/
|
positive charge |
e96 /eɲɔltɔ/ []
|
charge; ionization; ion |
e965 /eɲɔltɔlpɔ/ []
|
conducing S/m electrical conductivity ; σ |
e968 /eɲɔltɔrkɔ/ []
|
with electronegativity in Pauling scale |
e968ppo /eɲɔltɔrkɔpapo/ ⋄
|
2.20 |
e97 /eɲɔjɔ/ []
|
with component |
e98 /eɲɔrkɔ/ []
|
atomic mass |
e983 /eɲɔrkɔɲcɔ/ []
|
dense g/cm3 density |
e986 /eɲɔrkɔltɔ/ []
|
statistics |
e986b /eɲɔrkɔltɔba/
|
boson |
e986f /eɲɔrkɔltɔfa/
|
fermion |
e987 /eɲɔrkɔjɔ/ []
|
weighing standard atomic weight; relative atomic mass; Ar |
e98ops /eɲɔrkɔopasa/ ⋄
|
14 |
e99 /eɲɔɲɔ/ []
|
atomic number; Z ↞ dvt protons |
eU /euj/
|
metals ↞ eXbWo metals |
eX /exaw/
|
periods |
eXX /exawxaw/
|
groups; families |
eXXX /exawxawxaw/
|
isotopes |
eXXa /exawxawɛ/ []
|
charge; ionization; ion |
eXXaj /exawxawɛʒa/
|
---; -3 |
eXXaɭ /exawxawɛla/
|
--; -2 |
eXXam /exawxawɛma/
|
- |
eXXap /exawxawɛpa/
|
+ |
eXXaq /exawxawɛca/
|
++; +2 |
eXXar /exawxawɛra/
|
+++; +3 |
eXbWo /exawbawawo/
|
metals |
eXb /exawba/
|
alkali metals; group 1 elements |
eXc /exawʃa/
|
alkaline earth metals; group 2 elements |
eXdWm /exawdawawma/
|
transition metals |
eXd /exawda/
|
group 3 elements |
eXe /exawe/
|
group 4 elements |
eXf /exawfa/
|
group 5 elements |
eXg /exawga/
|
group 6 elements |
eXh /exawɣa/
|
group 7 elements |
eXi /exawi/
|
group 8 elements |
eXj /exawʒa/
|
group 9 elements |
eXk /exawka/
|
group 10 elements |
eXɭ /exawla/
|
group 11 elements |
eXm /exawma/
|
group 12 elements |
eXn /exawna/
|
boron group; group 13; 3 elements |
eXo /exawo/
|
carbon group; group 14; 4 elements |
eXpWs /exawpawawsa/
|
nonmetals |
eXp /exawpa/
|
nitrogen group; group 15; 5 elements |
eXq /exawca/
|
chalcogens; group 6; 16 elements |
eXr /exawra/
|
halogens; group 17; 7 elements |
eXs /exawsa/
|
noble gases; group 18; 8; 0 elements |
ean /eɛna/
|
nucleus; nuclide ↞ dv hadrons ≈ DDC 539 |
eao /eɛo/
|
orbitals; electronic clouds ≈ DDC 539 |
eaoX /eɛoxaw/
|
principal quantum number; n |
eaoXX /eɛoxawxaw/
|
azimuthal quantum number; angular momentum; l |
eaoXXX /eɛoxawxawxaw/
|
magnetic quantum number; orientation; m |
eaoXXXX /eɛoxawxawxawxaw/
|
spin quantum number; s |
eaoXXXXm /eɛoxawxawxawxawma/
|
s=-1/2 |
eaoXXXXp /eɛoxawxawxawxawpa/
|
s=+1/2 |
eaoXXXm /eɛoxawxawxawma/
|
-1/2 |
eaoXXXp /eɛoxawxawxawpa/
|
+1/2 |
eaoXXk /eɛoxawxawka/
|
-4 |
eaoXXɭ /eɛoxawxawla/
|
-3 |
eaoXXm /eɛoxawxawma/
|
-2 |
eaoXXn /eɛoxawxawna/
|
-1 |
eaoXXo /eɛoxawxawo/
|
0 |
eaoXXp /eɛoxawxawpa/
|
+1 |
eaoXXq /eɛoxawxawca/
|
+2 |
eaoXXr /eɛoxawxawra/
|
+3 |
eaoXXs /eɛoxawxawsa/
|
+4 |
eaoXb /eɛoxawba/
|
s orbitals; sharp; l=0 |
eaoXc /eɛoxawʃa/
|
p orbitals; principal; l=1 |
eaoXd /eɛoxawda/
|
d orbitals; diffuse; l=2 |
eaoXf /eɛoxawfa/
|
f orbitals; fundamental; l=3 |
eaoXg /eɛoxawga/
|
g orbitals; l=4 |
eaoXh /eɛoxawɣa/
|
h orbitals; l=5 |
eaoXi /eɛoxawi/
|
i orbitals; l=6 |
eaob /eɛoba/
|
level 1 orbitals; n=1 |
eaobb /eɛobaba/
|
1s subshell |
eaoc /eɛoʃa/
|
level 2 orbitals; n=2 |
eaocb /eɛoʃaba/
|
2s subshell |
eaocc /eɛoʃaʃa/
|
2p subshell |
eaoccn /eɛoʃaʃana/
|
m=-1 |
eaocco /eɛoʃaʃo/
|
m=0 |
eaoccp /eɛoʃaʃapa/
|
m=+1 |
eaod /eɛoda/
|
level 3 orbitals; n=3 |
eaoe /eɛoe/
|
level 4 orbitals; n=4 |
eaof /eɛofa/
|
level 5 orbitals; n=5 |
eaog /eɛoga/
|
level 6 orbitals; n=6 |
eaoh /eɛoɣa/
|
level 7 orbitals; n=7 |
eb /eba/
|
period 1 elements |
ebXX /ebaxawxaw/
|
period 1 isotopes |
ebb /ebaba/
|
hydrogen; H ↞ dt87nb dle87nb |
ebbap /ebabɛpa/
|
H+ |
ebbb /ebababa/
|
protium; 1H |
ebbc /ebabaʃa/
|
deuterium; 2H |
ebbd /ebabada/
|
tritium; 3H |
ebs /ebasa/
|
helium; He |
ebsd /ebasada/
|
helium-3; 3He |
ebse /ebase/
|
helium-4; 4He |
ec /eʃa/
|
period 2 elements |
ecXX /eʃaxawxaw/
|
period 2 isotopes |
ecb /eʃaba/
|
lythium; Li |
ecbap /eʃabɛpa/
|
Li+ |
ecc /eʃaʃa/
|
beryllium; Be |
ecn /eʃana/
|
boron; B |
eco /eʃo/
|
carbon; C |
ecoak /eʃoɛka/
|
C---- |
ecoas /eʃoɛsa/
|
C++++ |
ecoc /eʃoʃa/
|
carbon-12; 12C |
ecod /eʃoda/
|
carbon-13; 13C |
ecoe /eʃoe/
|
carbon-14; 14C |
ecp /eʃapa/
|
nitrogen; N |
ecpaɭ /eʃapɛla/
|
N--- |
ecpam /eʃapɛma/
|
N-- |
ecpan /eʃapɛna/
|
N- |
ecpf /eʃapafa/
|
nitrogen-15; 15N |
ecq /eʃaca/
|
oxygen; O |
ecqam /eʃacɛma/
|
O-- |
ecqan /eʃacɛna/
|
O- |
ecr /eʃara/
|
fluorine; F |
ecran /eʃarɛna/
|
F- |
ecs /eʃasa/
|
neon; Ne |
ed /eda/
|
period 3 elements |
edb /edaba/
|
sodium; Na |
edbap /edabɛpa/
|
Na+ |
edc /edaʃa/
|
magnesium; Mg |
edcap /edaʃɛpa/
|
Mg+ |
edcaq /edaʃɛca/
|
Mg++ |
edn /edana/
|
aluminium; aluminum; Al |
ednaq /edanɛca/
|
Al++ |
ednar /edanɛra/
|
Al+++ |
edo /edo/
|
silicon; Si |
edoak /edoɛka/
|
Si---- |
edoas /edoɛsa/
|
Si++++ |
edp /edapa/
|
phosphorus; P |
edpaɭ /edapɛla/
|
P--- |
edpb /edapaba/
|
phosphorus-31; 31P |
edq /edaca/
|
sulfur; sulphur; S |
edqam /edacɛma/
|
S-- |
edqan /edacɛna/
|
S- |
edr /edara/
|
chlorine; Cl |
edran /edarɛna/
|
Cl- |
eds /edasa/
|
argon; Ar |
ee /eje/
|
period 4 elements |
eeb /ejeba/
|
potassium; K |
eebap /ejebɛpa/
|
K+ |
eebk /ejebaka/
|
potassium-40; 40K |
eec /ejeʃa/
|
calcium; Ca |
eecap /ejeʃɛpa/
|
Ca+ |
eecaq /ejeʃɛca/
|
Ca++ |
eed /ejeda/
|
scandium; Sc |
eee /ejeje/
|
titanium; Ti |
eef /ejefa/
|
vanadium; V |
eeg /ejega/
|
chromium; Cr |
eeh /ejeɣa/
|
manganese; Mn |
eehaq /ejeɣɛca/
|
Mn++ |
eei /ejei/
|
iron; Fe |
eeiaq /ejeiɛca/ ⋄
|
Fe++ |
eeiar /ejeiɛra/ ⋄
|
Fe+++ |
eeih /ejeiɣa/
|
iron-57; 57Fe |
eej /ejeʒa/
|
cobalt; Co |
eek /ejeka/
|
nickel; Ni |
eeɭ /ejela/
|
copper; Cu |
eeɭaq /ejelɛca/
|
Cu++ |
eem /ejema/
|
zinc; Zn |
een /ejena/
|
gallium; Ga |
eeo /ejeo/
|
germanium; Ge |
eep /ejepa/
|
arsenic; As |
eeq /ejeca/
|
selenium; Se |
eer /ejera/
|
bromine; Br |
ees /ejesa/
|
krypton; Kr |
ef /efa/
|
period 5 elements |
efb /efaba/
|
rubidium; Rb |
efc /efaʃa/
|
strontium; Sr |
efd /efada/
|
yttrium; Y |
efe /efe/
|
zirconium; Zr |
eff /efafa/
|
niobium; Nb |
efg /efaga/
|
molybdenum; Mo |
efh /efaɣa/
|
technetium; Tc |
efi /efi/
|
ruthenium; Ru |
efj /efaʒa/
|
rhodium; Rh |
efk /efaka/
|
palladium; Pd |
efɭ /efala/
|
silver; Ag |
efm /efama/
|
cadmium; Cd |
efn /efana/
|
indium; In |
efo /efo/
|
tin; Sn |
efp /efapa/
|
antimony; Sb |
efq /efaca/
|
tellurium; Te |
efr /efara/
|
iodine; I |
efs /efasa/
|
xenon; Xe |
eg /ega/
|
period 6 elements |
egb /egaba/
|
caesium; Cs |
egc /egaʃa/
|
barium; Ba |
egcz /egaʃaza/
|
lanthanoids |
egczb /egaʃazaba/
|
lanthanum; La |
egczc /egaʃazaʃa/
|
cerium; Ce |
egczd /egaʃazada/
|
praseodymium; Pr |
egcze /egaʃaze/
|
neodymium; Nd |
egczf /egaʃazafa/
|
promethium; Pm |
egczg /egaʃazaga/
|
samarium; Sm |
egczh /egaʃazaɣa/
|
europium; Eu |
egczi /egaʃazi/
|
gadolinium; Gd |
egczj /egaʃazaʒa/
|
terbium; Tb |
egczk /egaʃazaka/
|
dysprosium; Dy |
egczɭ /egaʃazala/
|
holmium; Ho |
egczm /egaʃazama/
|
erbium; Er |
egczn /egaʃazana/
|
thulium; Tm |
egczo /egaʃazo/
|
ytterbium; Yb |
egd /egada/
|
lutetium; Lu |
ege /ege/
|
hafnium; Hf |
egf /egafa/
|
tantalum; Ta |
egg /egaga/
|
tungsten; W |
egh /egaɣa/
|
rhenium; Re |
egi /egi/
|
osmium; Os |
egj /egaʒa/
|
iridium; Ir |
egk /egaka/
|
platinum; Pt |
egɭ /egala/
|
gold; Au |
egm /egama/
|
mercury; Hg |
egn /egana/
|
thallium; Tl |
ego /ego/
|
lead; Pb |
egp /egapa/
|
bismuth; Bi |
egq /egaca/
|
polonium; Po |
egr /egara/
|
astatine; At |
egs /egasa/
|
radon; Rn |
eh /eɣa/
|
period 7 elements |
ehb /eɣaba/
|
francium; Fr |
ehc /eɣaʃa/
|
radium; Ra |
ehcz /eɣaʃaza/
|
actinoids |
ehczb /eɣaʃazaba/
|
actinium; Ac |
ehczbf /eɣaʃazabafa/
|
actinium-230; 230Ac |
ehczc /eɣaʃazaʃa/
|
thorium; Th |
ehczcf /eɣaʃazaʃafa/
|
thorium-230; 230Th |
ehczcg /eɣaʃazaʃaga/
|
thorium-231; 231Th |
ehczd /eɣaʃazada/
|
protactinium; Pa |
ehcze /eɣaʃaze/
|
uranium; U |
ehczef /eɣaʃazefa/
|
uranium-235; 235U |
ehczej /eɣaʃazeʒa/
|
uranium-239; 239U |
ehczf /eɣaʃazafa/
|
neptunium; Np |
ehczg /eɣaʃazaga/
|
plutonium; Pu |
ehczh /eɣaʃazaɣa/
|
americium; Am |
ehczi /eɣaʃazi/
|
curium; Cm |
ehczj /eɣaʃazaʒa/
|
berkelium; Bk |
ehczk /eɣaʃazaka/
|
californium; Cf |
ehczɭ /eɣaʃazala/
|
einsteinium; Es |
ehczm /eɣaʃazama/
|
fermium; Fm |
ehczn /eɣaʃazana/
|
mendelevium; Md |
ehczo /eɣaʃazo/
|
nobelium; No |
ehd /eɣada/
|
lawrencium; Lr |
ehe /eɣe/
|
ruthefordium; Rf |
ehf /eɣafa/
|
dubnium; Db |
ehg /eɣaga/
|
seaborgium; Sg |
ehh /eɣaɣa/
|
bohrium; Bh |
ehi /eɣi/
|
hassium; Hs |
ehj /eɣaʒa/
|
meitnerium; Mt |
ehk /eɣaka/
|
darmstadtium; Ds |
ehɭ /eɣala/
|
roentgenium; Rg |
ehm /eɣama/
|
copernicium; Cn |
ehn /eɣana/
|
ununtrium; Uut |
eho /eɣo/
|
flerovium; Fl |
ehp /eɣapa/
|
ununpentium; Uup |
ehq /eɣaca/
|
livermorium; Lv |
ehr /eɣara/
|
ununseptium; Uus |
ehs /eɣasa/
|
ununoctium; Uuo |
Connected classes:
|
⌕ damq
|
chemical energy ↞ e |
⌕ e96 [eXXa]
|
charge; ionization; ion |
⌕ e97 [eao]
|
with component |
⌕ eU
|
metals ↞ eXbWo |
⌕ f
|
molecules; chemical substances; pure substances [chemistry; molecular physics] ↞ e |
⌕ faei
|
reduction-oxidation; redox ↞ ecq |
⌕ faoh
|
hydrogen bonding ↞ ebb |
⌕ fc
|
mineral acids; inorganic acids ↞ ebb f99f |
⌕ fccWf
|
hydrogen halides ↞ eXr |
⌕ fcc
|
hydrofluoric acid; HF ↞ ecr |
⌕ fcd
|
hydrochloric acid; HCl ↞ edr |
⌕ fce
|
hydrobromic acid; HBr ↞ eer |
⌕ fcf
|
hydroiodic acid; HI ↞ efr |
⌕ fcm
|
nitric acid; HNO3 ↞ ecp |
⌕ fcn
|
nitrous acid; HNO2 ↞ ecp |
⌕ fco
|
halogen oxoacids ↞ eXr |
⌕ fcp
|
phosphoric acid; H3PO4 ↞ edp |
⌕ fcq
|
sulfuric acid; sulphuric acid; oil of vitriol; H2SO4 ↞ edq |
⌕ fcr
|
fluorosulfuric acid; HSO3F ↞ ecr edq |
⌕ fdd
|
ammonia; azane; NH3 ↞ ecp |
⌕ fdhc
|
lithium hydroxide; LiOH ↞ ecb |
⌕ fdhd
|
sodium hydroxide; NaOH ↞ edb |
⌕ fdhm
|
magnesium hydroxide; Mg(OH)2 ↞ edc |
⌕ fe
|
oxides ↞ ecq |
⌕ feb
|
hydrogen oxides ↞ ebb |
⌕ fec
|
carbon oxides ↞ eco |
⌕ fed
|
nitrogen oxides; NOx ↞ ecp |
⌕ fee
|
lithium oxide; lithia; Li2O ↞ ecb |
⌕ fef
|
sodium oxide; Na2O ↞ edb |
⌕ feg
|
magnesium oxide; magnesia; MgO ↞ edc |
⌕ feh
|
aluminium oxides ↞ edn |
⌕ fei
|
silicon oxides ↞ edo |
⌕ fek
|
potassium oxide; potash; K2O ↞ eeb |
⌕ feɭ
|
calcium oxides ↞ eec |
⌕ fen
|
manganese oxides ↞ eeh |
⌕ fer
|
iron oxides; rust ↞ eei |
⌕ fev
|
copper oxides ↞ eel |
⌕ few
|
zinc oxide; ZnO ↞ eem |
⌕ ffcWf
|
halides ↞ eXr |
⌕ ffc
|
fluorides ↞ ecr |
⌕ ffd
|
chlorides ↞ edr |
⌕ ffe
|
bromides ↞ eer |
⌕ fff
|
iodides ↞ efr |
⌕ ffk
|
carbonates ↞ eco |
⌕ ffm
|
nitrates ↞ ecp |
⌕ ffn
|
nitrites ↞ ecp |
⌕ ffp
|
phosphates ↞ edp |
⌕ ffq
|
sulfates ↞ edq |
⌕ fh
|
hydrocarbons ↞ eco ebb |
⌕ fɭ
|
carboxylic acids; fatty acids ↞ fc ebb |
⌕ ibbc
|
native carbon ↞ eco |
⌕ ibbi
|
native iron ↞ eei |
⌕ ibbɭ
|
native gold ↞ egl |
⌕ ibc
|
sulfide minerals; sulphides; sulfides ↞ edq |
⌕ ibcgg
|
galena; PbS ↞ ego |
⌕ ibchc
|
cinnabar; HgS ↞ egm |
⌕ ibchh
|
sphalerite; ZnS ↞ eem |
⌕ ibcpp
|
pyrite; FeS2 ↞ eei |
⌕ ibcxm
|
molybdenite; MoS2 ↞ efg |
⌕ ibd
|
sulfosalt minerals ↞ edq |
⌕ ibeb
|
ice; H2O ↞ ebb fU |
⌕ ibecb
|
periclase; MgO ↞ edc |
⌕ ibeec
|
corundum; Al2O3 ↞ edn fehd |
⌕ ibeee
|
hematite; Fe2O3 ↞ eei ferd |
⌕ ibekc
|
cassiterite; SnO2 ↞ efo |
⌕ ibekp
|
pyrolusite; MnO2 ↞ eeh |
⌕ ibekr
|
rutile; TiO2 ↞ eee |
⌕ ibesc
|
chromite; FeCr2O4 ↞ eeg eei |
⌕ ibesm
|
magnetite; Fe3O4 ↞ edc eei fere |
⌕ ibess
|
spinel; MgAl2O4 ↞ edc edn |
⌕ ibf
|
hydroxide minerals; hydroxides ↞ ecq ebb |
⌕ ibh
|
halide minerals; halides ↞ eXr |
⌕ ibhff
|
fluorite; CaF2 ↞ ecr eec |
⌕ ibhhh
|
table salt; halite; NaCl ↞ edr edb |
⌕ ibhhs
|
sylvite; KCl ↞ edr eeb |
⌕ ibm
|
carbonate minerals; carbonates ↞ eco ecq |
⌕ ibmc
|
calcite group ↞ eec |
⌕ ibmid
|
dolomite; CaMg(CO3)2 ↞ edc eec |
⌕ ibn
|
nitrate minerals; nitrates ↞ ecp |
⌕ ibo
|
sulphate minerals; sulphates; sulfates ↞ edq |
⌕ ibobb
|
barite; BaSO4 ↞ egc |
⌕ ibobc
|
celestine; SrSO4 ↞ efc |
⌕ ibobn
|
anglesite; PbSO4 ↞ ego |
⌕ iboqg
|
gypsum; CaSO4·2H2O ↞ eec |
⌕ ibp
|
phosphate minerals; phosphates ↞ edp |
⌕ ibpif
|
fluorapatite; Ca5(PO3)3F ↞ ecr |
⌕ ibpih
|
chlorapatite; Ca5(PO4)3Cl ↞ edr |
⌕ ibr
|
borate minerals; borates ↞ ecn |
⌕ ibs
|
silicates ↞ edo |
⌕ ibsbdo
|
olivine; (Mg;Fe2+)2[SiO4] ↞ edc eei |
⌕ ibspo
|
kaolinite-serpentine group ↞ edc eei |
⌕ ibsptc
|
talc ↞ edc |
⌕ ibx
|
organic minerals ↞ eco |
⌕ idgp
|
pearl ↞ mq eco ecq |
⌕ idgs
|
silicate glasses ↞ edo |
⌕ vahg
|
copper technology; Copper Age; chalcolithic ↞ eel |
⌕ vahh
|
bronze technology; Bronze Age ↞ eel efo |
⌕ vahi
|
iron technology; Iron Age; steel ↞ eei |
⌕ vde
|
metal mining ↞ eU |
⌕ wafh
|
metal; metallic ↞ eU vde |
⌕ yisd
|
physics ↞ b c d e g |